Introduction:
Circulating tumor DNA (ctDNA) has emerged as a tool to characterize tumors and track minimal residual disease (MRD) in many malignancies, including diffuse large B-cell lymphoma (DLBCL). ctDNA levels have been shown as prognostic after first-line treatment and CD19-targeted chimeric antigen receptor (CAR19) T cell therapy in DLBCL. Loncastuximab tesirine (loncastuximab tesirine-lpyl [Lonca]), an antibody-drug conjugate comprising an antibody targeting CD19 and a pyrrolobenzodiazepine dimer cytotoxin, is approved for relapsed/refractory (R/R) DLBCL. The ultrasensitive ctDNA-MRD detection method, phased variant enrichment and detection sequencing (PhasED-Seq) was applied to evaluate molecular response and mutational genotypes in patients (pts) undergoing Lonca treatment.
Methods:
LOTIS-2 study (NCT03589469) evaluated the efficacy of Lonca in R/R DLBCL after ≥ 2 lines of prior systemic therapy. Samples from 33 LOTIS-2 pts were profiled by PhasED-Seq (Foresight Diagnostics), representing a range of best responses to therapy, including 8 complete response (CR), 14 partial response (PR), and 11 progressive disease (PD). Pts received a median of 4 treatment cycles with Lonca (range 2-22). Baseline plasma and peripheral blood mononuclear cells were used to identify tumor-specific phased variants (PVs), which were used to monitor ctDNA-MRD after 1 cycle of treatment (cycle 2, day 1 [C2D1]) and at the end of treatment (EOT). Samples were reported quantitatively with levels of ctDNA-MRD and qualitatively as ctDNA-MRD positive or negative. Absolute ctDNA levels and log-fold change (LFC) in ctDNA were compared with outcomes including best overall response, progression-free survival (PFS), and overall survival (OS), as determined by the independent review committee. The ctDNA mutational profile of each pt was evaluated at baseline and EOT to assess emerging clonal alterations with emphasis on CD19.
Results:
PVs were successfully genotyped from pretreatment plasma in 31/33 pts (94%) with sufficient material for analysis. The median pretreatment ctDNA level was 141 haploid genome equivalents /mL (hGE/mL) (range 0.4-3608), similar to previous DLBCL studies in first-line and relapsed settings. Interestingly, pretreatment ctDNA levels were not predictive of outcomes to treatment (hazard ratio [HR] 0.99, P=0.96).
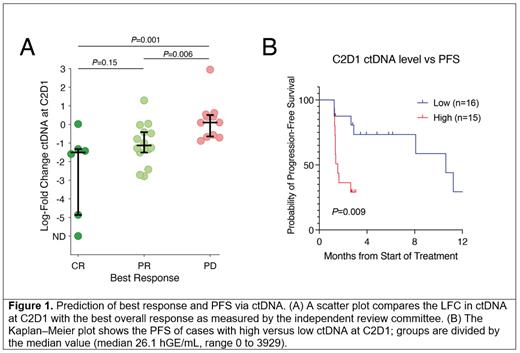
The prognostic value of ctDNA levels was assessed at C2D1. Absolute ctDNA levels and the change from baseline levels were significantly prognostic for the response to treatment. Pts achieving either a CR or a PR had significantly greater reduction in their ctDNA levels than those failing to respond (CR vs PD, median LFC -1.5 vs 0.1, P=0.001; PR vs PD, median LFC -1.1 vs 0.1, P=0.006) (Fig 1A). Interestingly, pts achieving a CR or a PR did not have significantly different changes in ctDNA at C2D1 ( P=0.15). When dividing pts at the median into those with high vs low ctDNA levels at C2D1, lower levels of ctDNA were significantly associated with superior PFS and OS (PFS: HR 4.4, P=0.009; OS: HR 3.2, P=0.01) (Fig 1B).
At the EOT, 5 of 6 CR pts had cleared their ctDNA-MRD to undetectable levels vs 1 of 6 at C2D1. The CR pt who did not show deepening of molecular response was the only CR pt who ended Lonca treatment for radiographic disease progression; all other CR pts ended treatment for other reasons (e.g., toxicity, transplant, or persistent complete remission).
To assess for emergent clonal mutations, the mutational profiles of pts were evaluated before Lonca treatment and at EOT. Prior studies in DLBCL after CAR19 T-cell therapy have revealed recurrent, although infrequent, mutations in CD19 as a mechanism of resistance. In this cohort, no emergent alterations in CD19 at the EOT (0/31 pts) were observed. One pt was identified with a baseline mutation in CD19 (R363C); this pt achieved a PR followed by PD after 3 cycles of treatment with persistence of the mutation in CD19.
Conclusions:
ctDNA molecular response assessment using PhasED-Seq is prognostic for outcomes in pts receiving Lonca monotherapy. ctDNA levels as early as C2D1 can predict outcomes and are indicative of a fast response to Lonca. Furthermore, molecular responses can deepen with additional cycles. CD19 alterations do not appear to be a common emergent mechanism of resistance to Lonca. In this exploratory study, ctDNA-MRD predicts Lonca efficacy and outcomes and should be further considered as a universal biomarker in DLBCL.
Disclosures
Kurtz:Foresight Diagnostics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties: Patents Pertaining to circulating tumor DNA licensed to Foresight Diagnostics. Hogan:Foresight Diagnostics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Schultz:Foresight Diagnostics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Chabon:Foresight Diagnostics: Current Employment, Current equity holder in private company. Alizadeh:Roche: Consultancy, Honoraria, Other: Travel, accommodations and expenses; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Stanford University: Patents & Royalties: ctDNA detection; Gilead Sciences: Consultancy, Other: Travel, accommodations and expenses; Celgene: Consultancy, Research Funding; CiberMed: Consultancy, Current holder of stock options in a privately-held company; CAPP Medical: Current holder of stock options in a privately-held company; Forty Seven: Current holder of stock options in a privately-held company; Lymphoma Research Foundation: Consultancy; Syncopation Life Sciences: Current holder of stock options in a privately-held company; Janssen Oncology: Honoraria. Havenith:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Genmab. Samari:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company. Kopotsha:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company. Wang:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company, Other: holder of ADC Therapeutics stock options. Qin:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company. Wang:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company. Pantano:ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company, Other: holder of ADC Therapeutics stock options; Novartis: Current equity holder in publicly-traded company, Other: spouse is current employee/equity holder, Patents & Royalties: coinventor of Novartis patent; Alcon: Current equity holder in publicly-traded company, Other: spouse is current equity holder; Merck & Co, Organon: Current equity holder in publicly-traded company.